CATEGORIES
Metallic vs. Poisoned Target
When sputtering a metallic target in a reactive atmosphere there is an interesting effect that must be accounted for. Metals have a relatively large sputter erosion rate and the high conductivity of the target avoids the build up of charge, enabling efficient bombarding from the plasma without a counteracting electric field. The sputtering rate is high for a given field strength.
If the desired coating is an oxide or nitride of the metal, it is customary to use a metallic target for the metallic component and add the reactive gas (oxygen, nitrogen or a mixture of both) to the argon plasma. This process is called reactive sputtering as opossed to the process in which the target itself is already a compound. The coating of an insulator is associated with a relatively low deposition rate at a given sputter field strength. Besides, the use of a AC field becomes mandatory. ECR plasma sputter tools use an RF field for sputtering and deal perfectly well will both metallic and insulating targets.
The ECR plasma coating tool uses metallic targets and adds reactive gas to create insulating thin film coatings.
Metallic Target Mode
At very low reactive gas partial pressure, the whole quantity of the reactive gas is virtually immediately gettered by the sputtered metal in the gaseous phase and incorporated into the thin film. This effect is so efficient, that not even an increase in the background pressure is noticeable. In this stage the deposition rate remains high. However, the resulting thin film with these deposition conditions may be unsatisfactory because the proportion of metal to oxide or nitride is far from stoichiometric. For optical films this translates in a large absorption coefficient (the layer is not transparent, because of the strong metallic character). It is necessary to increase the reactive gas flow to achieve a stoichiometric compound.
Yet, when we increase the reactant gas flow to make sure that all metallic sputtered species are gettered, we come dangerously close to the hysteresis shaped cliff of the poisoned target mode. See below for more explanations.
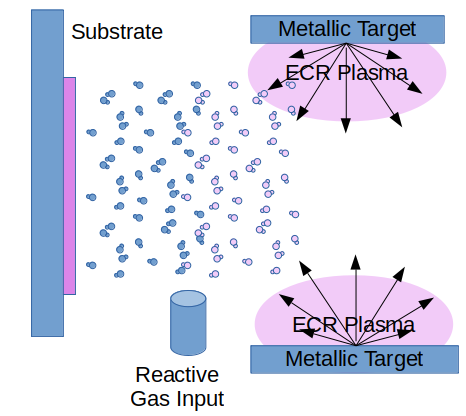
Taking the risk of a thin film with a slight metallic character, the deposition rate is kept high. The addition of reactive gas is low enough. The gas input is chemically absorbed by the sputter gas virtually to 100% and is completely incorporated into the film.
Poisoned Target Mode
Yet what happens next when increasing the reactive gas flow, is that this gas is not completely absorbed by the metal-vapor species, but a part of it reaches the target. On the target, it is quickly adsorbed, creating a few nanometer thick surface layer of oxide or nitride on top of the metallic target. The so-called “poisoning” of the target is an an avalanche-like transition that renders the target an electric insulator and even in an RF sputter field, much tougher to sputter: The sputter erosion rate of the target significantly and suddenly drops, depriving the inflowing reactive gas from sufficient reaction partners. This translates in a sudden and significant increase in the background pressure, because the reactive gas is not immediately gettered and incorporated into the growing thin film but becomes part of the background pressure. Films grown with the target in poisoned mode are grown with lower deposition rate but their properties are closer to a stoichiometric thin film. It is easy to ensure, that the deposited species are 100% saturated with the reactive gas. There is no disadvantage in an oversupply with reactive gas besides the comparatively much lower deposition rate.
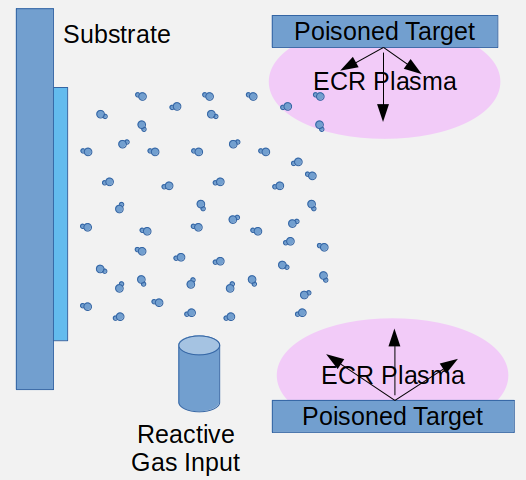
For low absorption optical thin films, the growing film must be completely saturated by reactive gas components. What happens here as a consequence of the increased reactive gas flow is a insulating layer covering the target and a sudden drop in the growth rate and an increase in the background pressure.
Hysteresis
Since the target has been poisoned and the sputter erosion rate is now so low, there is an oversupply of reactive gas that increases the background pressure of the chamber. Reducing its flow to the point at which the poisoning of the target happened, does not have any significant effect. It is necessary to considerably overcompensate to make the reactive gas so rare, that the insulating layer of the target can be sputtered away and become metallic again.
This reverse effect is sudden as well: When the poisoning layer of the target is cleaned away, the sudden increase of the sputter erosion rate and the sudden over-availability of “unpartnered” metallic species in the chamber leads to a sudden drop in the background pressure because the reactive species find more than enough metallic species to partner with.
This hysteretic behavior of reactive sputtering makes its control a bit challenging.
Ideally, what we want is to keep the target in the metallic mode to make sure that the deposition rate is very high. And at the same time we want to make sure, that virtually all metal atoms are fully saturated with a reactant. The ECR plasma sputter system is so easy to control, that exactly this is possible. With an ECR plasma sputter system from JSW Afty, you can deposit insulating films with a non-insulating target in the high-deposition-rate metallic mode while achieving fully stoichiometric conditions.
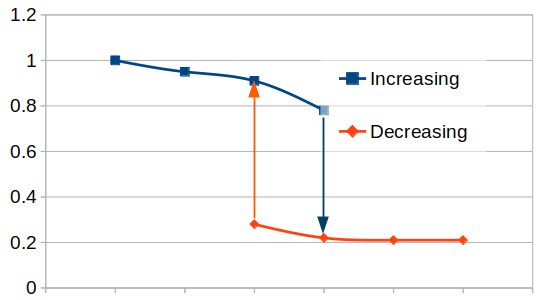
By increasing the reactive gas flow (x-axis), the sputter erosion rate (y-axis, a.u.) suddenly drops. Decreasing the rate does not render the same behavior until well past the former gas flow parameter.
Do you like what you see?
We value your feedback, so let us know what you think!
Let us also know which topics you would like to see expanded.
Just give us a call, send us an e-mail or use the form to contact us.
